| Kidney Res Clin Pract > Volume 34(1); 2015 > Article |
|
Abstract
Background
The patency of arteriovenous access is important for stable and effective hemodialysis, and long-term technical survival is best achieved with a native arteriovenous fistula (AVF). However, maintaining AVF patency remains a challenge. This study was designed to determine the independent prognostic factors for AVF patency according to hemodialysis duration.
Methods
The primary study end point was unassisted patency of the AVF, which was defined as the time from the first fistula surgery to the first AVF failure. AVF failure was defined as an event that required percutaneous intervention or surgery to revise or replace the fistula, which occurred at least 2 months after fistula formation.
Results
We enrolled 478 patients with a mean age of 55.5±14.0 years, and mean duration of dialysis was 2.5±2.1 years. There were 109 cases (22.8%) of AVF failure. The factors related to AVF patency differed according to hemodialysis duration. Using a Cox-adjusted model, we observed a significant correlation between the incidence of AVF failure and diabetes within the initial 12 months of hemodialysis. Uncontrolled hyperphosphatemia (mean serum phosphorus>5.5 mg/dL during hemodialysis) was associated with patency loss of AVF after 1 year of hemodialysis.
Conclusion
Various factors were associated with the development of patency loss of AVF as hemodialysis duration differed, and a preventive role of hyperphosphatemia control in AVF survival needs further clinical study.
Keywords
Arteriovenous fistula, Hemodialysis, HyperphosphatemiaThe survival of arteriovenous fistula (AVF) remains an important problem for hemodialysis patients, accounting for ~20% of all hospitalizations related to AV access problems in western countries. After a continuous effort to increase the placement of autologous AVF in the National Kidney Foundation’s original Dialysis Outcomes Quality Initiative (DOQI), the prevalence of AVF reached up to 55% in March 2010 [1]. Mean facility use of fistulas in Japan, however, was already 92% in 2003. This difference in AVF placement rate resulted in higher AVF survival and primary failure occurred in only 7.6% of fistulas, and 70% of these were recorded in Japan [2]. The importance of AVF placement is that once the suitability for dialysis has been achieved, fistula has a better survival compared with AV grafts. The use of AVF is related with fewer access complications compared with AV grafts, as well as low risk of infection, hospitalization, and death compared with AV grafts and catheters.
The clinical risk factors associated with AV access failure are advanced age, diabetes mellitus, poor surgical technique, previous catheter insertion, and history of peripheral vascular diseases [3,4]. AVF patency is involved with specific genetic polymorphism in transforming growth factor β1, methylene tetrahydrofolate reductase, angiotensin-converting enzyme and length of heme oxygenase-1 [5–8]. We previously reported that angiotensin-converting enzyme polymorphism is associated with AVF survival, and serum phosphorus level is also a clinical factor related to AVF survival [8]. The genetic polymorphism is not a correctable factor, while serum phosphorus level is possible to be controlled in dialysis patients. If we can establish the modifiable risk factors related to hemodialysis, there is substantial room for improvement of AVF survival. Therefore, we explored the modifiable risk factors between loss of AVF patency according to hemodialysis duration and clinical characteristics of patients.
We enrolled end-stage renal disease (ESRD) patients who were treated with maintenance hemodialysis in 2010 at five university or teaching hospitals (Kyung Hee University Hospital, Kyung Hee University Hospital at Gangdong, Konkuk University, Hanyang University Guri Hospital, National Health Insurance Ilsan Hospital) and whose complete medical records were available retrospectively. Patients were included if they met the following criteria: (1) creation of a native AVF as the first vascular access irrespective of temporary catheter use for hemodialysis; (2) formation of the AVF by similar surgical methods with end-to-side anastomosis; and (3) treatment with hemodialysis for 4 h, three times weekly. We excluded patients based on the following criteria: (1) if they received an AV graft with synthetic material as the first vascular access; (2) access failure occurred within the first 2 months after fistula surgery; and (3) if the first episode of AVF failure was related to an infectious complication, steal phenomenon or aneurysm.
Patients’ medical records were used for compiling clinical data retrospectively, including age, gender, underlying causes of ESRD, history of ipsilateral central venous catheter insertion, and the side (right or left) and location (forearm or upper arm) of the AVF. The causes of ESRD were categorized as diabetes mellitus, hypertension, glomerulonephritis, others, or unknown. Laboratory findings, such as serum hemoglobin, serum calcium, phosphate, and intact parathyroid hormone (PTH) were obtained from the medical records. The mean laboratory levels were calculated from the time of the initial AVF occurrence to an AVF event. The primary study end point was unassisted patency of the AVF, which was defined as the time from the first fistula surgery to the first patency loss of AVF. Patency loss of AVF was defined as an event that required percutaneous intervention or surgery to revise or replace the fistula, which occurred at least 2 months after fistula formation. The early event was defined as patency loss of AVF within 1 year of the start of hemodialysis, and the late event was defined as patency loss of AVF after 1 year of hemodialysis. Additionally, patients were censored at the time of kidney transplantation, or if they were transferred to other hospitals, and death with a functioning AVF. The duration between AVF operation and HD initiation can be differed depending on the patient's condition but this difference was not considered in this study.
Continuous variables were expressed as mean±standard deviation. One-way analysis of variance or the Student t test was used to compare the continuous variables of the groups. The categorical variables were expressed as counts and percentages and analyzed using the χ2 test. The survival curve for the unassisted AVF patency was assessed by the Kaplan–Meier method and compared using the log-rank test. The Cox proportional hazard regression model that allowed adjustment for various baseline differences (age and sex) was used to confirm the independent prognostic factors associated with the patency loss of AVF.
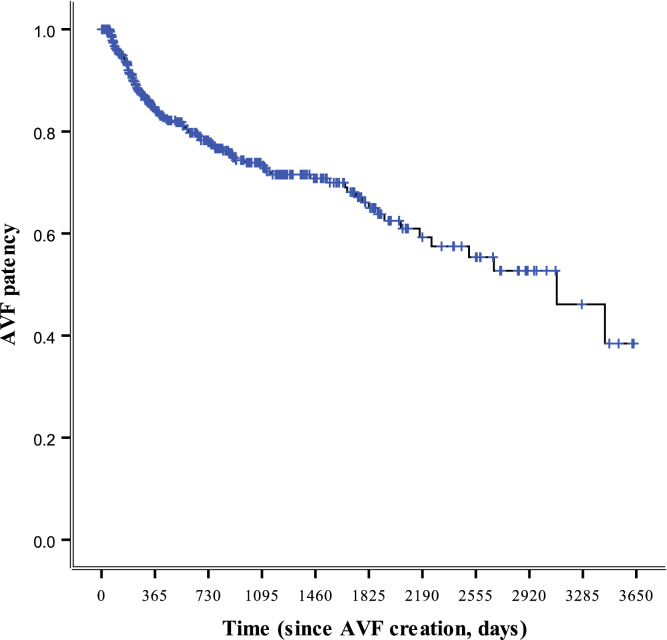
A total of 478 hemodialysis patients with ESRD were included in this study. Table 1 shows the demographic features, vascular access, and clinical characteristics of all the study patients. The mean age of the population was 55.5±14.0 years. The causes of ESRD were diabetic nephropathy (52.3%), hypertension (22.6%), glomerulonephritis (7.1%), miscellaneous (8.4%), and unknown (9.6%). A left-sided AVF was recorded in 426 (89.2%) patients, and a right-sided AVF was present in 52 (10.8%) patients. The forearm and upper arm for the AVF location was noted in 351 (73.4%) and 127 (26.6%) cases, respectively. A temporary central catheter was replaced in 183 (38.3%) patients. The mean dialysis duration was 2.5±2.1 years. The unassisted patency of AVF were 85%, 74% and 66% at 1 year, 3 years and 5 years since AVF creation, respectively (Fig. 1). Patency loss was reported in 109 (22.8%) AVFs during the study period.
The patients with patency loss of AVF had a significantly higher prevalence of type 2 diabetes mellitus, mean serum phosphorus level>5.5 mg/dL, and mean calcium×phosphorus product>55 (mg2/dL2, P<0.05; Table 2). To determine the relationship between strict phosphorus control and AVF survival, patients were classified into two groups according to mean serum phosphorus level above or below 5.5 mg/dL, based on the K-DOQI-recommended level [9]. No significant difference was observed between age, gender, number of temporary central catheters used, the site or location of the AVF, mean serum calcium, and mean intact PTH. There was no significant difference in medication use between the two groups.
The unassisted patency of the AVF was significantly lower in diabetic mellitus patients than in nondiabetic patients (P<0.01, Fig. 2A). The patients with mean serum phosphorus level>5.5 mg/dL also showed lower unassisted patency of AVF (P<0.05, Fig. 2B). Diabetes mellitus and mean serum phosphorus level differed significantly according to hemodialysis duration.
Among the 109 cases of patency loss of AVF, 62 occurred within 1 year of hemodialysis. We explored the risk factors associated with patency loss of AVF before and after 1 year of hemodialysis. Table 3 shows the Cox regression model for association of AVF failure with the selected parameters. In the early event, the unassisted patency of the AVF was significantly lower in diabetes mellitus than in nondiabetic patients (Table 3, hazard ratio=2.37, P=0.002). In case of the late event, the group with a mean serum phosphorus level below 5.5 mg/dL had a higher AVF survival rate than the group with a mean serum phosphorus level above 5.5 mg/dL (Table 3, hazard ratio=2.17, P=0.009).
Most risk factors for AVF failure have already been determined at the time of surgery, and there are few studies about risk factors associated with patency loss of AVF according to the duration of hemodialysis. The risk factors during continuous hemodialysis, such as vascular calcification, repeated puncture, and vascular changes in diabetes mellitus may be related to the patency loss of AVF. In this study, we demonstrated that known risk factors of patency loss of AVF were related to early patency loss of AVF. Uncontrolled hyperphosphatemia, however, was related to late patency loss of AVF.
We analyzed clinical risk factors of primary AVF events before and after 1 year. Other recent studies also reported that low intra access blood flow (<500 mL/min), diabetes, older age, and smoking history are clinical risk factors of primary AVF event within 6 months [10]. During this study, we presumed that AVF survival would differ according to dialysis period. Therefore, various factors which might influence AVF survival were explored by 6-month period. We confirmed that different factors from those of the early stage affected AVF survival at 1 year after initiation of dialysis. Previous studies reported diabetic patients are at risk of increased primary AVF failure and reduced primary patency rate [11,12]. In this study, diabetes was also an independent risk factor for patency loss of AVF within 1 year. It was interesting that the role of diabetes as a risk factor for AVF survival decreased after the initial 1 year.
The Dialysis Outcomes and Practice Patterns Study (DOPPS) showed the difference in fistula placement between Europe and the US [13]. Furthermore, both fistula and graft survival were superior in Europe in the DOPPS analysis adjusted for case-mix [14,15]. A recent study reported the reasons for these differences in access selection and survival associated with surgical technique. The probability of AVF failure was significantly lower for vascular access surgeons who placed≥25 fistulae during training [16]. In general, the creation of an AV access is considered as low priority for surgeons. For this reason, creation of vascular access is not a procedure that many surgical residents are trained to perform, and only a few have sufficient experience to perform it well. However, surgical technique may influence AVF survival. A limitation of this study was that we did not evaluate the influence of surgical technique.
Hyperphosphatemia is closely associated with vascular calcification. Hyperphosphatemia and an elevated Ca×P product are related to cardiovascular mortality in patients with chronic kidney disease [9,17,18]. For the dialysis patients, vascular calcification is associated with hypercalcemia, hyperphosphatemia, elevated Ca×P product, and ingested oral calcium [19–21]. Medial artery calcification in which amorphous mineral forms circumferentially along or within one or more elastic lamellae of the medial layer is more prevalent in patients with diabetes and chronic kidney disease [22]. Most studies determining vascular calcification have been performed in vascular smooth cells, which are the main component of the medial arterial layer. Many key regulators of bone formation and bone structural proteins are expressed in both calcified medial arterial layers and atherosclerotic plaques. Sage et al [23] reported that elevated levels of phosphorus in cultured smooth muscular cells induce expression of osteogenic genes, such as bone morphogenetic protein and osteopontin. Hyperphosphatemia is more closely associated with all-cause and cardiovascular mortality than serum calcium level and iPTH [24]. It is associated with the fact that increased serum phosphate or phosphate overload may directly promote vascular injuries, including vascular calcification, arterial stiffness, endothelial dysfunction, and left ventricular hypertrophy [25]. Previous studies indicated that the high mean serum phosphorus level was associated with worse vascular access survival [26,27]. These studies reported an association between risk of vascular access thrombosis and increased serum phosphorus, Ca×P product, and PTH levels in hemodialysis patients. Previous studies included both native and polytetrafluoroethylene graft, however, we enrolled only native AVF to avoid confusion.
A limitation of this study was that we only evaluated the clinical factors related to AVF survival. Other important factors such as the status of native vein also may influence the patency of AVF.
The present study demonstrated that patency loss of AVF in 478 hemodialysis patients was related to the presence of diabetes and the causative factor within the first year of hemodialysis. Uncontrolled phosphorus level (mean serum phosphorus level>5.5 mg/dL) was the most important factor in AVF failure at 1 year after hemodialysis. The different risk factors were associated with patency loss of AVF according to the hemodialysis duration, and a preventive role of hyperphosphatemia control for AVF survival needs further study.
References
1. Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI. Dialysis Access Consortium Study Group. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA 299:2008;2164–2171.



2. Ohira S, Kon T, Imura T. Evaluation of primary failure in native AV-fistulae (early fistula failure). Hemodial Int 10:2006;173–179.


3. Erkut B, Unlu Y, Ceviz M, Becit N, Ateş A, Colak A, Koçak H. Primary arteriovenous fistulas in the forearm for hemodialysis: effect of miscellaneous factors in fistula patency. Ren Fail 28:2006;275–281.


4. Woods JD, Turenne MN, Strawderman RL, Young EW, Hirth RA, Port FK, Held PJ. Vascular access survival among incident hemodialysis patients in the United States. Am J Kidney Dis 30:1997;50–57.


5. Fukasawa M, Matsushita K, Kamiyama M, Mikami Y, Araki I, Yamagata Z, Takeda M. The methylentetrahydrofolate reductase C677T point mutation is a risk factor for vascular access thrombosis in hemodialysis patients. Am J Kidney Dis 41:2003;637–642.


6. Heine GH, Ulrich C, Köhler H, Girndt M. Is AV fistula patency associated with angiotensin-converting enzyme (ACE) polymorphism and ACE inhibitor intake? Am J Nephrol 24:2004;461–468.


7. Heine GH, Ulrich C, Sester U, Sester M, Köhler H, Girndt M. Transforming growth factor beta1 genotype polymorphisms determine AV fistula patency in hemodialysis patients. Kidney Int 64:2003;1101–1107.


8. Moon JY, Jeong KH, Paik SS, Han JJ, Lee SH, Lee TW, Ihm CG, Kim MJ, Chung . Arteriovenous fistula patency associated with angiotensin-converting enzyme I/D polymorphism and ACE inhibition or AT1 receptor blockade. Nephron Clin Pract 111:2009;c110–c116.


9. Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: recommendations for a change in management. Am J Kidney Dis 35:2000;1226–1237.


10. Monroy-Cuadros M, Yilmaz S, Salazar-Bañuelos A, Doig C. Risk factors associated with patency loss of hemodialysis vascular access within 6 months. Clin J Am Soc Nephrol 5:2010;1787–1792.



11. Huijbregts HJ, Bots ML, Moll FL, Blankestijn PJ; CIMINO members. Hospital specific aspects predominantly determine primary failure of hemodialysis arteriovenous fistulas. J Vasc Surg 45:2007;962–967.


12. Huijbregts HJ, Bots ML, Wittens CH, Schrama YC, Moll FL, Blankestijn PJ; CIMINO study group. Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative. Clin J Am Soc Nephrol 3:2008;714–719.



13. Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis 44:2004;7–15.

14. Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, Wolfe RA, Goodkin DA, Held PJ. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int 61:2002;305–316.


15. Saran R, Dykstra DM, Pisoni RL, Akiba T, Akizawa T, Canaud B, Chen K, Piera L, Saito A, Young EW. Timing of first cannulation and vascular access failure in haemodialysis: an analysis of practice patterns at dialysis facilities in the DOPPS. Nephrol Dial Transplant 19:2004;2334–2340.


16. Saran R, Elder SJ, Goodkin DA, Akiba T, Ethier J, Rayner HC, Saito A, Young EW, Gillespie BW, Merion RM, Pisoni RL. Enhanced training in vascular access creation predicts arteriovenous fistula placement and patency in hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study. Ann Surg 247:2008;885–891.


17. Marco MP, Craver L, Betriu A, Belart M, Fibla J, Fernández . Higher impact of mineral metabolism on cardiovascular mortality in a European hemodialysis population. Kidney Int Suppl Jun:2003;S111–S114.

18. Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int 67:2005;1179–1187.


19. Ekim M, Yüksel S, Fitöz S, Ozmert E, Acar B, Ozçakar ZB, Güvence N, Atalay S, Yalçinkaya F. Systemic vascular calcification with retinal calcification in an adolescent treated with long-term peritoneal dialysis. Pediatr Nephrol 21:2006;1915–1916.


20. Goldsmith DJ, Covic A, Sambrook PA, Ackrill P. Vascular calcification in long-term haemodialysis patients in a single unit: a retrospective analysis. Nephron 77:1997;37–43.


21. Nitta K, Akiba T, Uchida K, Kawashima A, Yumura W, Kabaya T, Nihei H. The progression of vascular calcification and serum osteoprotegerin levels in patients on long-term hemodialysis. Am J Kidney Dis 42:2003;303–309.


22. Goodman WG, London G, Amann K, Block GA, Giachelli C, Hruska KA, Ketteler M, Levin A, Massy Z, McCarron DA, Raggi P, Shanahan CM, Yorioka N; Vascular Calcification Work Group. Vascular calcification in chronic kidney disease. Am J Kidney Dis 43:2004;572–579.


23. Sage AP, Lu J, Tintut Y, Demer LL. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int 79:2011;414–422.


24. Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52:2008;519–530.


25. Kendrick J, Chonchol M. The role of phosphorus in the development and progression of vascular calcification. Am J Kidney Dis 58:2011;826–834.



Figure 2
Kaplan–Meier analysis of AVF patency. Data according to (A) DM versus non-DM and (B) mean serum phosphorus level. AVF, arteriovenous fistula; DM, diabetes mellitus.
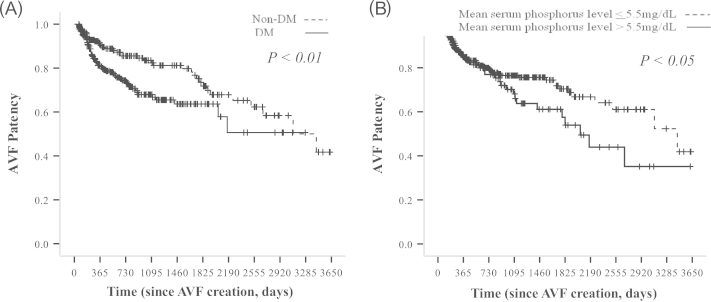
Table 1
Demographic, vascular access and clinical characteristics of patients (n=478)
Table 2
Clinical characteristics according to patency loss of AVF
Table 3
Independent factors prognostic of the AVF survival according to the hemodialysis duration⁎
| Factor |
Early event |
Late event |
||
|---|---|---|---|---|
| Hazard ratio | P-value | Hazard ratio | P-value | |
| Unadjusted Cox model | ||||
| Male sex | 1.04 | 0.87 | 0.57 | 0.05 |
| Age | 1.01 | 0.46 | 1.01 | 0.83 |
| Diabetic mellitus | 2.37 | 0.002 | 1.19 | 0.66 |
| Ischemic heart disease | 1.12 | 0.25 | 1.27 | 0.27 |
| Mean serum phosphorus level > 5.5 mg/dL | 1.20 | 0.71 | 1.85 | 0.03 |
| Mean serum calcium level > 9.5 mg/dL | 1.01 | 0.99 | 1.04 | 0.94 |
| Mean serum Ca x P > 55 | 1.10 | 0.83 | 1.63 | 0.33 |
| Mean serum iPTH > 300 | 1.04 | 0.84 | 1.21 | 0.21 |
| Multivariate adjusted Cox model† | ||||
| Diabetic mellitus | 2.37 | 0.002 | 1.60 | 0.132 |
| Mean serum phosphorus level > 5.5 mg/dL | 1.20 | 0.710 | 2.17 | 0.009 |



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















